Polymers
Polymers are large molecules built from small units (monomers). Different polymers are built from different monomers and have varying linkages between the monomers.
Synthetic polymers
Synthetic polymers are man-made polymers such as nylon (polyamide) and terylene (polyester).
These two polymers will be covered in more detail down below
There are two main methods of polymerization:
- Addition polymerization
- Condensation polymerization
Both nylon and tetrylene are made via condensation polymerization.
Addition polymerization
This have been covered briefly in the topicalkenes.
By breaking apart the double bond of alkenes, repeating units (monomers) can be joined together into a larger, longer molecule (polymer).
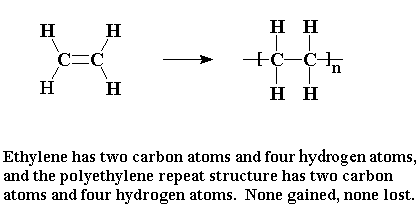
The contents inside the square brackets indicate the repeating unit (monomer) with the letter ‘n’ indicating the total number of the monomers throughout the structure. Take notice of the fact that the double bond has become a single bond in the polymer.
Condensation polymerization
In a condensation reaction, two monomers react together and join. During the reaction a water molecule is lost in the process (therefore condensation).
Polyamides (nylon)
In polyamides (such as nylon) the two monomers are always a dicarboxylic acid (or diacid) and a diamine. These two monomers join together via an amide link as shown below:
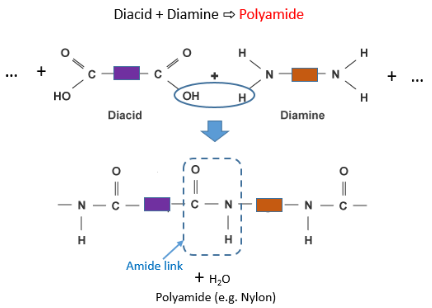
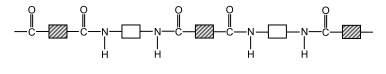
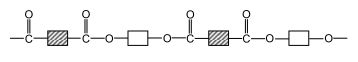
Here is the official structure of nylon as drawn in the CIE syllabus:
Polyesters (tetrylene)
In polyesters (such as tetrylene) the two monomers are a diacid and a diol. These monomers are held via an ester link as shown below:
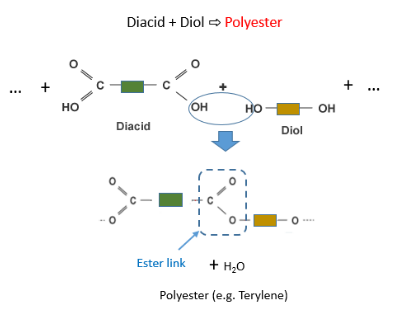
Here is the official structure of tetrylene as drawn in the CIE syllabus:
Plastics and pollution
Polymers are non-biodegradable, meaning that they do not decay. They are a major source of visual pollution and fill up available waste sites. Burning them isn’t good because poisonous gases form as a result. The best long-term solution to disposal would be to recycle polymer waste.
Natural polymers
Proteins and carbohydrates are important constituents of food. These molecules are natural polymers that you find in the body.
Proteins
Proteins are built from amino acid monomers that are joined via amide links (through condensation polymerization) – Exactly like nylon but different units!

Here is the official structure of protein in the CIE syllabus:
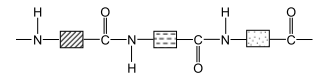
There are many different types of amino acids (up to 20) that can be found. Each block above demonstrates a different amino acid.
As above, amino acids can be joined together via condensation polymerization to form proteins. Meanwhile, proteins can then be hydrolyzed back to amino acids by boiling with hydrochloric acid.

Paper chromatography can be useful in determining what amino acids are present after hydrolyzing the protein.
Carbohydrates
Complex carbohydrates (polysaccharides) are made from a large number of simple sugar units that are joined via condensation polymerization.
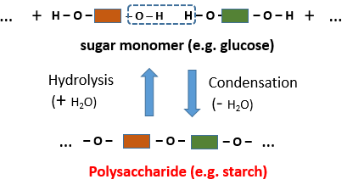
Here is the official structure of complex carbohydrates in the CIE syllabus:
![]()
Complex carbohydrates can also be hydrolyzed back into simple sugars by heating with dilute hydrochloric acid. This reaction can also be catalyzed by enzymes.