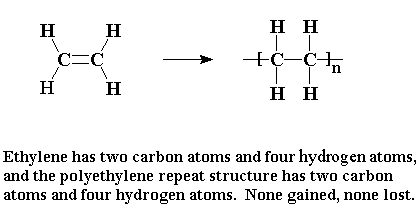Properties of alkenes
Alkenes have a general formula:
![]()
Alkenes contain carbon double bonds (C=C). Unlike alkanes, alkenes can undergo addition reactions due to the C=C double bond, and are therefore called unsaturated hydrocarbons.
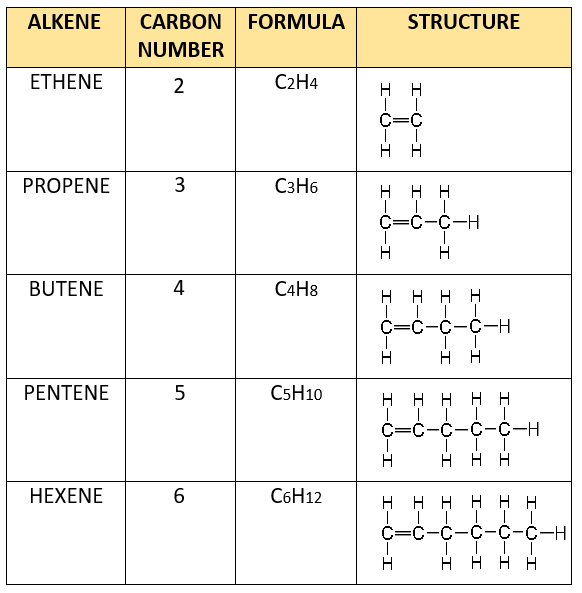
Alkene homologous series
Alkene manufacture
Alkenes are made by cracking alkanes. Large alkane molecules obtained by fractional distillation of petroleum, are passed over a heated catalyst (silicon IV oxide & aluminium oxide).
The idea is that larger alkanes can be broken down into simpler alkanes, alkenes, and possibly hydrogen
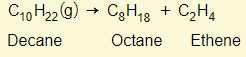
But there is more than just one possibility of products. For example:
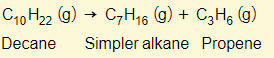
Hydrogen can also be made as well:
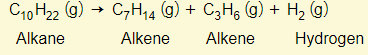
Alkene reactions
Addition reactions
The C=C double bond in alkenes can be broken to “add” molecules onto the compound. There will therefore two reactants but only one product formed.
Alkenes can undergo addition reactions with bromine, hydrogen, and steam.
Bromine (test for alkenes)
Aqueous bromine undergoes addition reactions with alkenes. As a result, the original brown colour of aqueous bromine will turn colourless in the presence of alkenes.
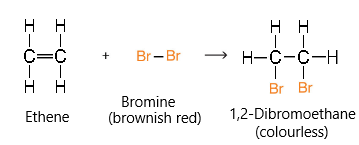
Notice how the C=C double bond in the ethene/ethylene molecule becomes broken and bromine atoms add onto each of the respective carbons.
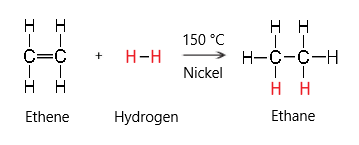
Hydrogen
Hydrogen reacts with alkenes to produce alkanes.
The conditions required for this reaction are:
- Temperature 150 degrees
- Nickel catalyst
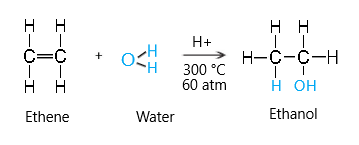
Water (steam)
Water can react with alkenes to make alcohols. This type of reaction is called hydration.
The conditions required for this reaction are:
- Temperature 300 degrees
- Pressure 60 atmospheres
- Phosphoric acid catalyst
Addition polymerization
Polymerization is the formation of long chain molecules called polymers froma large number of monomer molecules. down below shows addition polymerization because there is only one product – The polymer.