Metallic properties
Physical properties
We have touched on the physical properties of metals in the previous topics. Here is a brief summary:
- Shiny
- Good conductors of heat/electricity
- High density
- Malleable and ductile
- Usually solid at room temperature
- Sonorous (makes bell-like sounds when struck)
Chemical properties
Metal + Acid
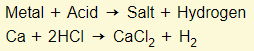
Metal + Oxygen
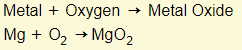
Metal + Cold Water
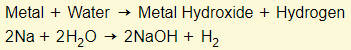
Metal + Steam
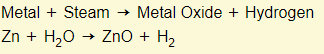
Note: Reactive metals such as sodium and potassium will react fine in cold water to produce hydroxide salts. Less reactive metals like copper will not react in cold water. They will only react in steam and produce oxide salts instead of hydroxide salts.
Alloys
An alloy is a mixture of two or more metals, or a mixture of one or more metals with a non-metal. Alloys are used in preference to pure metals because they can be designed to have properties for whatever usage purpose. For example, they may be made to be harder and more resistant to corrosion.
Alloys are harder than pure metals because the presence of different sized atoms will make the layers less mobile and prevent them from slipping.
This diagram below represents a simple alloy: The mixture of metallic atoms (red) with other different atoms (blue):
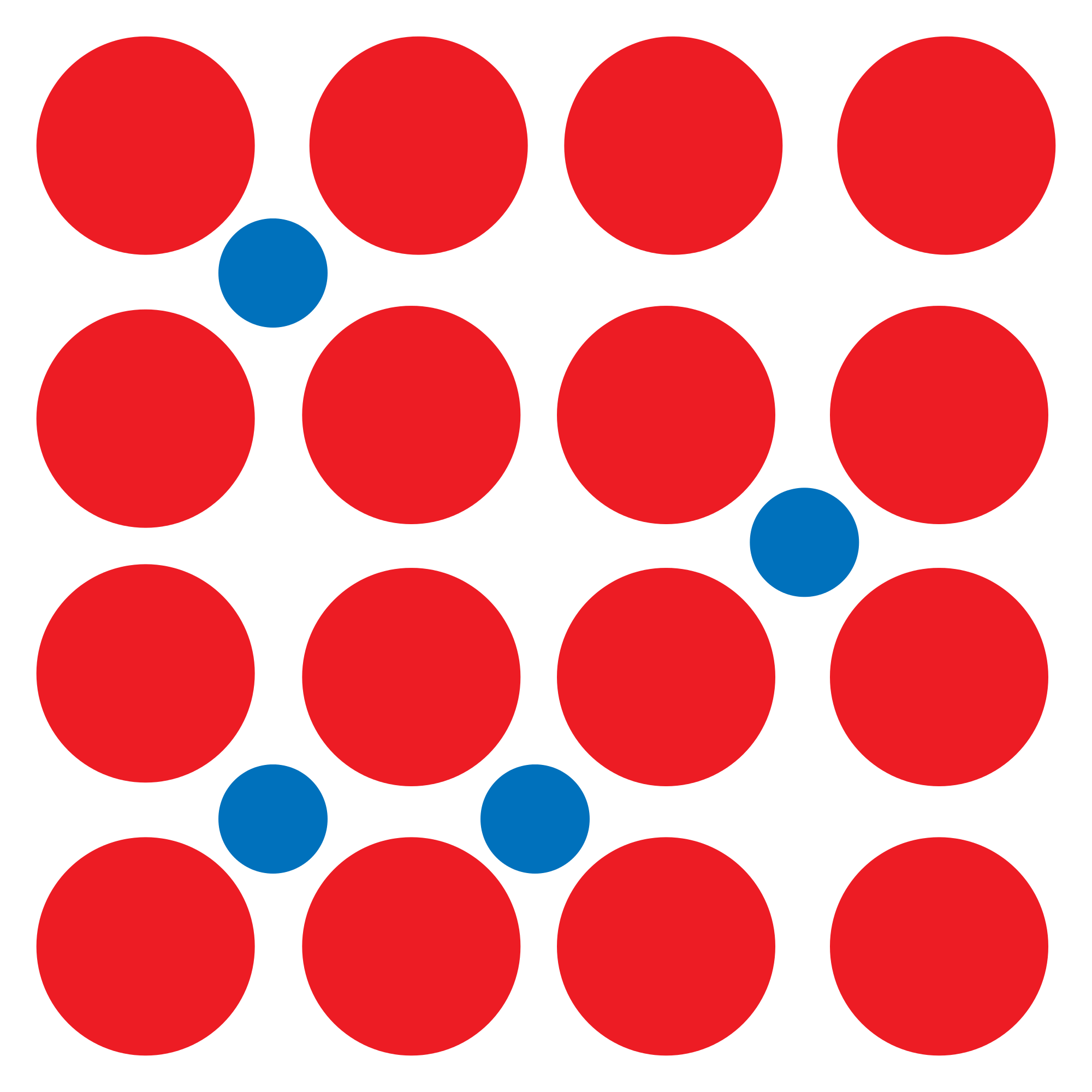
Some examples of alloys are:
- Brass: mixture of zinc and copper
- Mild steel: Iron and up to 0.3% carbon
- Stainless steel: Iron, nickel, chromium
Reactivity series
Different metals have varying reactivities. This all dependent on the tendency of a metal to form its positive ion. The greater the tendency to form the ion, the greater the reactivity of the metal.
The reactivity series orders metals from most reactive to least reactive:
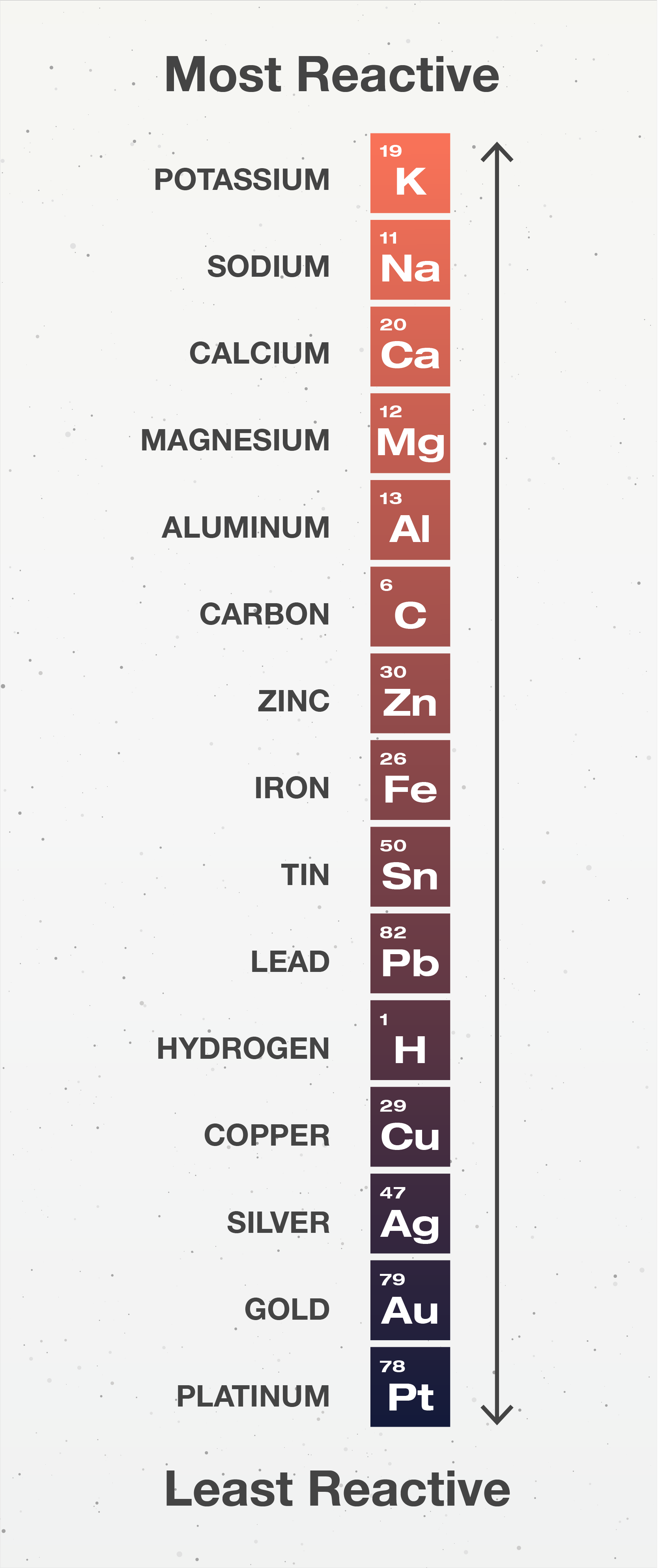
NOTE: The main ones you need to know are potassium, sodium, calcium, magnesium, zinc, iron, hydrogen, and copper
The reactivity series tells us that potassium (highest in series) atoms have a much higher tendency to become cations than, say, platinum (lowest), and therefore much more reactive.
We can demonstrate the difference in reactivities by observing the reactions of each of these metals with steam, dilute acid, and also the reduction of the oxides with carbon.
Remember, we have looked at metal + water (cold/steam) & acid reactions above.
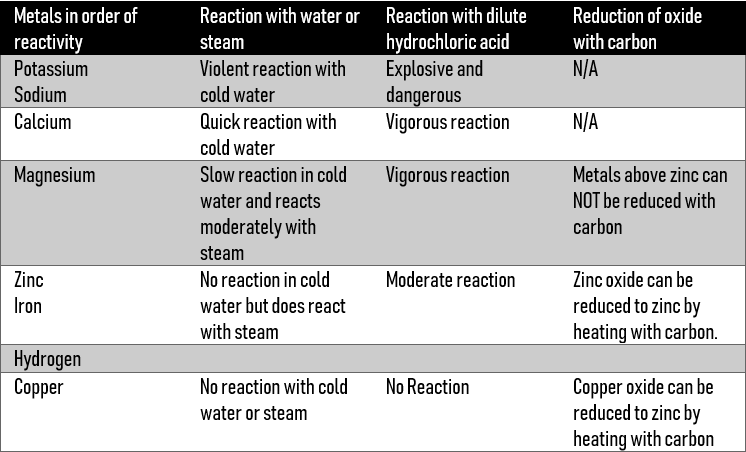
As you can see, the reactions become less vigorous down the table, suggesting the reduction of reactivities of the metals.
Reduction of metal oxides via carbon
Remember, reduction is the loss of oxygen. If a metal oxide gets “reduced” by carbon, it means that carbon “steals” an oxygen from the metal oxide. For example:
![]()
The rule is, only a more reactive element can “steal” an oxygen from an oxide.
In this scenario, zinc oxide is reduced by carbon because carbon is more reactive than zinc (refer to the reactivity series).
This is why the metal oxides that have metals above carbon on the reactivity series can not be reduced but those that are below it can.
Displacement reactions
As mentioned above, the reactivity series is based upon the metal’s tendency to become cations. The greater the tendency, the greater its reactivity.
Displacement reactions involve one ion replacing another. Whether or not a metal can displace another metal in a compound is strictly dependent on their relative reactivities.
Example 1
![]()
Examine the reaction above. What has happened to each of the metals in the equation?
The zinc has changed from the zinc metal to the ion form (in zinc sulfate). Meanwhile, the copper ions (in copper sulfate) has become copper metal:
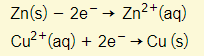
This is a redox reaction. Zinc loses 2 electrons (oxidation) and donates them to the copper ions that ultimately gain the 2 electrons (reduction).
Quite simply, the more reactive metal zinc has displaced the copper in copper sulfate. This displacement occurs because of the transfer of electrons in the redox equation above.
Example 2
![]()
In this example, zinc can NOT displace the magnesium in magnesium chloride because it’s tendency to form ions is lower (i.e. less reactive).
Decomposition reactions (via heat)
A decomposition reaction occurs when one reactant breaks down into two or more products. The more reactive the metal, the more stable its compounds are (and thus harder to decompose).
Metal hydroxides
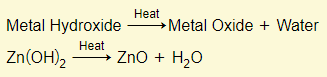
NOTE: Sodium and potassium hydroxides are exceptions and do not decompose when heated.
Metal nitrates
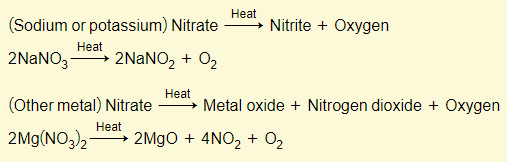
NOTE: Sodium and potassium nitrates decompose to nitrite + oxygen. Other metal nitrates however, decompose to the metal oxide, nitrogen dioxide, and oxygen.
Metal carbonates
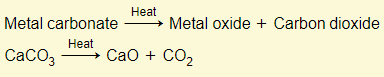
Extraction of metals
Reactive metals such as potassium, sodium, calcium, magnesium, and aluminium are extracted from their ores via electrolysis of a molten compound.
CIE requires you to understand the extraction of zinc, iron, and aluminium.
Zinc extraction
- The ore is zinc blende (ZnS)
- This is roasted in the air to form the oxide
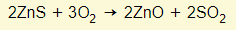
- The oxide is heated with carbon in a furnace, where it is reduced to zinc
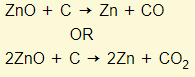
- Zinc distills out of the furnace
Iron extraction
- The ore is called haematite
- Haematite, coke (carbon), and limestone are added to a furnace
- Carbon dioxide is formed
- From reaction between coke and oxygen
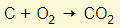
- From decomposition of limestone
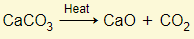
- From reaction between coke and oxygen
- Carbon dioxide gets reduced to carbon monoxide
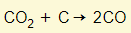
- Carbon monoxide reduces the iron (III) oxide to iron
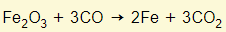
- The impurity in the ore is sand (silivon IV oxide)
- This reacts with calcium oxide to form slag
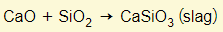
- Molten slag floats on molten iron
- This can be run off separately and used as building material
- This reacts with calcium oxide to form slag
Aluminium extraction
The details of aluminium extraction has been covered in the topic of electrolysis. Please click here and scroll down the page to find the relevant information.
NOTE: Aluminium is a reactive metal, and quite often it reacts with oxygen in the air to form a ‘aluminium oxide coating’. This oxide coating makes the metal seem unreactive.
Uses of metals
Here we go through some brief uses of several different metals
- Aluminium
- Aircraft manufacture due to strength and low density
- Food contains due to corrosion resistance
- Zinc
- Galvanizing and brass making
- Copper
- Electrical wiring and utensils
- Steel
- Car bodies and machinery
- Stainless steel