Energetics of a reaction
Definitions
All chemical reactions fall into two catergories: Exothermic or endothermic.
An exothermic reaction gives out heat. The total chemical energy of the reactants is larger than the products. This difference in energy is transferred to surroundings as heat.
An endothermic reaction takes in heat. The chemical energy of the reactants is smaller than the products so this difference in energy is transferred from the surroundings to the chemicals.
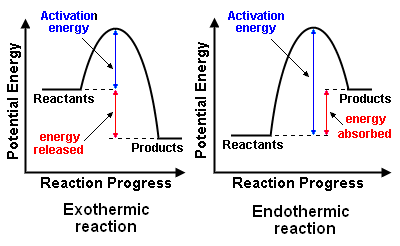
*The activation energy is the minimum quantity of energy which the reacting species must possess in order to undergo a specified reaction.
Bond breaking and bond making
The process of bond breaking is an endothermic process. Energy must be “taken in” to break bonds apart.
The process of bond making is an exothermic process. Energy (heat) is released when this happens.
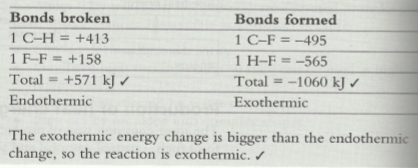
Quite often they will give you an equation such as:
![]()
They will also give you the relevant bond energies:
- C-H = 413 kJ/mol
- F-F = 158 kJ/mol
- H-F = 565 kJ/mol
- C-F = 495 kJ/mol
*An endothermic reaction has a (+) sign because energy is taken in. An exothermic reaction has a (-) sign because energy is lost. For instance, if you break one mole of C-H bonds it is denoted as +413. If you form one mole of C-H bonds, then it is denoted as -413.
From the information above, can you figure out whether the overall reaction is exothermic or endothermic?
Answer:
Energy transfer
The most common way of producing heat energy is by burning fossil fuels – natural gas, coal, petroleum products
Hydrogen as fuel
The combustion of hydrogen is highly exothermic. It is only used as a rocket fuel, in experimental vehicles, and fuel cells. There are advantages and disadvantages of hydrogen fuel.
- Advantages
- Very energy rich
- No pollutants are formed
- Nitrogen oxides not formed (these are environmentally harmful i.e. acid rain)
- Disadvantages
- Expensive to produce
- Difficult (and expensive) to store
Hydrogen fuel cells
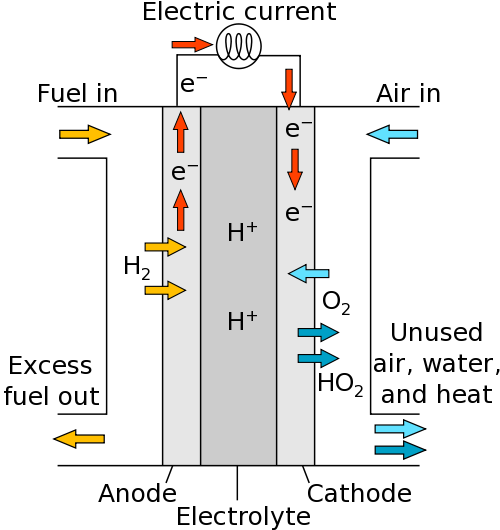
Much like the set-up for electrolysis, you have an anode (+) and cathode (-). Reactants are supplied to each electrode. The fuel (containing hydrogen) is supplied to the anode and the air (containing oxygen) is supplied to the cathode.
At the anode, hydrogen molecules lose electrons to form ions in the electrolyte:
![]()
At the cathode, oxygen gains electrons:
![]()
The ions formed react to product water:
![]()
The overall equation is:
![]()
The overall equation above demonstrating the formation of water is essentially an exothermic reaction (i.e. energy is released).
When hydrogen reacts with oxygen to form water in a fuel cell, electrical energy is produced.