Atomic structure
Atoms are the smallest particle of a chemical element that can exist. Elements are substances that are composed of just a single type of atom.
For example, the element carbon is made of only carbon atoms. Likewise, the element oxygen is made of only oxygen atoms. You get the gist.
The structure of an atom is made up of three sub-atomic particles: Protons, neutrons, and electrons.
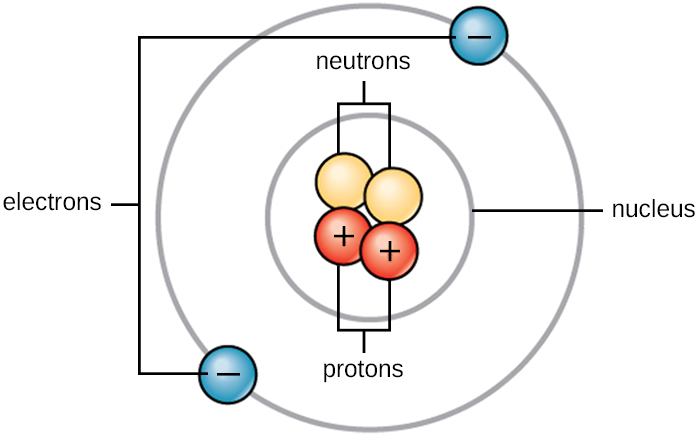
The above diagram is an example of a helium atom. There are several important things that you need to know about these sub-atomic particles.
- Location
- Protons & neutrons are always found in the nucleus
- Electrons are found in shells, and they orbit the nucleus
- Charge
- Protons have positive charge (+)
- Neutrons have zero charge (0)
- Electrons have negative charge (-)
- Mass
- Protons have a relative mass of 1
- Neutrons have a relative mass of 1
- Electrons have a negligible relative mass of 1/1840, which is essentially zero.
The table below is a summary:
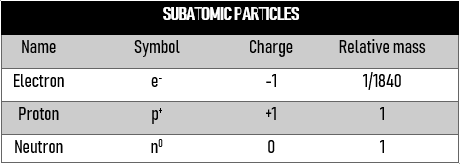
Isotopes are atoms with the same proton number (i.e. same element) but have different neutron numbers.
Periodic table
There are many chemical elements on earth, and the periodic table summarizes all of them into a single table:
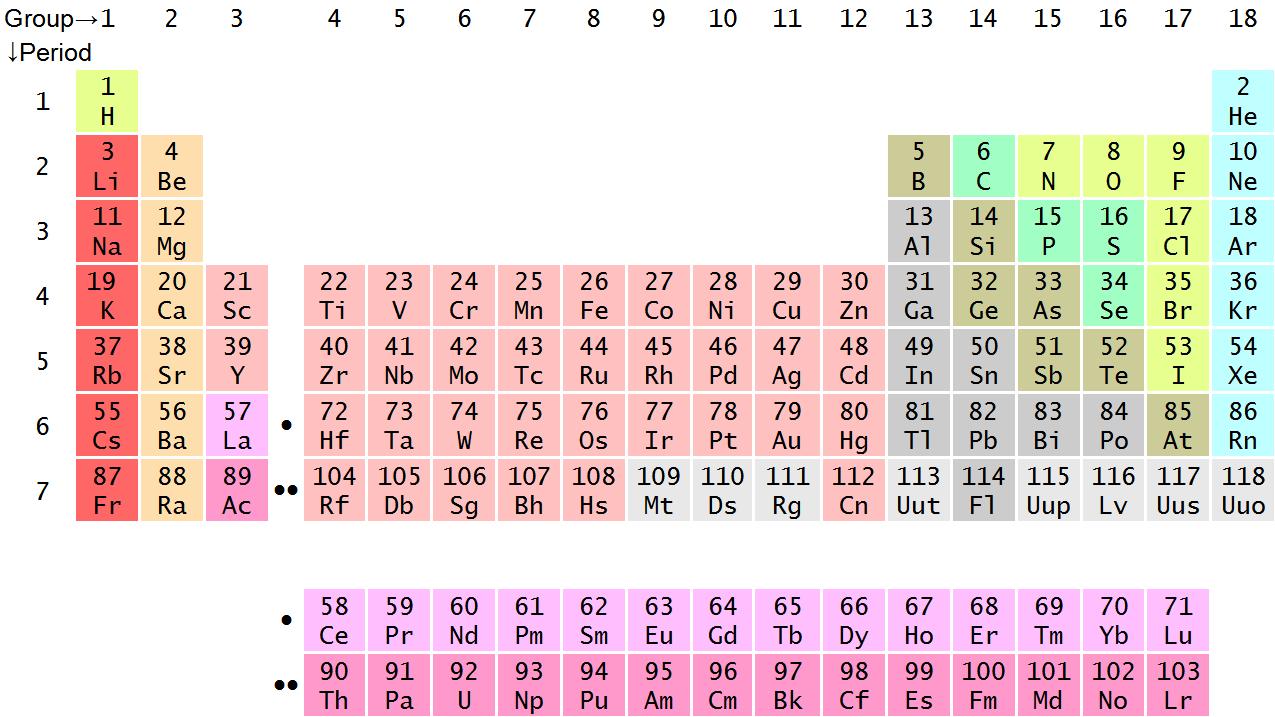
Not only does it tell us the full names of all existing elements (and their respective shortened symbols), but it also gives us important information regarding the structure of a single atom of that particular element.
Take helium for example:
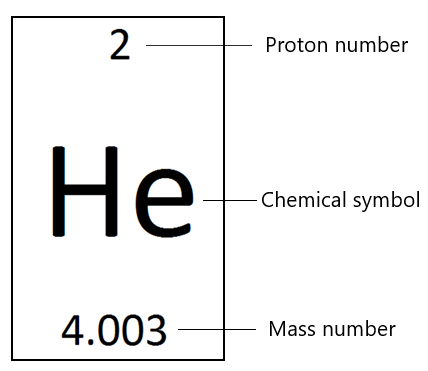
Firstly, it tells you the full name and shortened chemical symbol for the element. In this case – Helium (He). This is fairly straight forward.
Secondly, it tells you the proton number and the mass number of an helium atom. Here are a couple of extremely important things to remember:
- The proton number (aka atomic number) is the number of protons in the atom
- The mass number is the number total number of protons and neutrons (recall that neutrons/protons have mass but electrons do not)
- The number of electrons will always equal the number of protons
An atom always has zero overall charge because the number of protons (+) will always be canceled out by the same number of electrons (-). If there was an imbalance, then the atom would become charged and at that point, it is not called an atom. Instead, it is called an ion. This will be covered down below
This means that just from the information provided by the periodic table, you can calculate the number of protons, neutrons and electrons of the atoms of any particular element!
Lets take oxygen as an example this time:
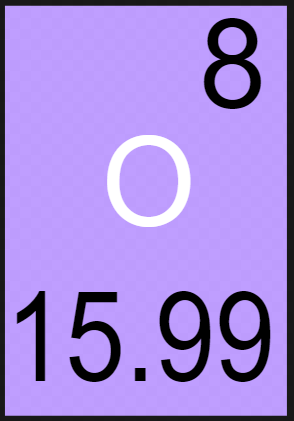
- An oxygen has 8 protons
- This means that it has 8 electrons
- Since its mass number (proton + neutron) is 16 it must mean that it has 8 neutrons
Electron arrangement
Recall that electrons are held in shells. Shells are represented as rings around the nucleus.
It is really important understand that the maximum number of electrons that a single shell can hold can vary.
Take a look at this diagram below:
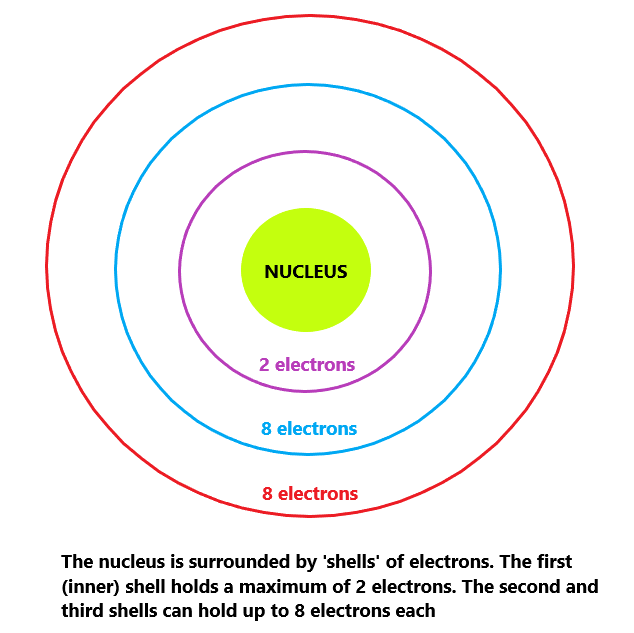
As described above, the first (inner) shell can hold up to 2 electrons. The second and third shell can hold up to 8 electrons.
It is important to realize that electrons fill from the most inner shell
Example: Oxygen
So lets take oxygen as an example again. Above, we established that a single oxygen atom holds 8 protons, 8 neutrons and 8 electrons.
Remember, electrons always fill from the inner shell first. Since we know that the first electron shell can only hold up to 2 electrons, it must mean that the rest of the electrons (6 of them) are held in the 2nd shell. This is what an oxygen atom therefore would look like diagrammatically:
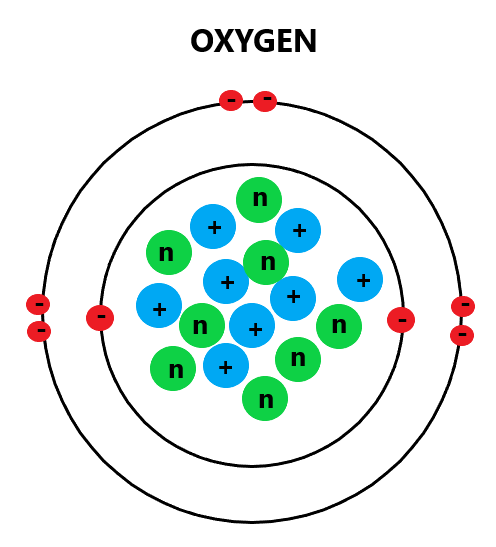
Knowing the electron arrangement of atoms are extremely important because it defines the entire foundation of chemistry. Why do you think atoms react with one another? It’s because all atoms have a goal. Do you know what that goal is? It’s simple: To achieve a full outer shell of electrons.
Reactivity of elements
As mentioned above, all atoms have a simple goal of wanting to achieve a full outer shell of electrons. If you look at the diagram of oxygen above, you will see that an oxygen atom has 6 electrons in its most outer shell. So have a think about it… how could oxygen achieve its goal? There are possible two main ways:
- Gain 2 electrons
- If an oxygen atom just added two extra electrons into its outer shell, then it would have 8, and therefore a full outer shell!
- Lose 6 electrons
- If an oxygen atom lost all six of its outer electrons, then that shell would simply disappear. That means the inner shell (with two electrons) will become the most “outer shell”. This would also mean that indeed the atom would now have a full outer shell since two electrons is the maximum (for that shell)!
You need to ask yourself. Would option 1 be easier or option 2? Indeed, gaining 2 is a lot easier than losing 6 and therefore this is what happens in reality. Oxygen either gains 2 extra electrons by sharing with other atoms or by a transfer process. This will be covered in detail down below (chemical bonding).
So really, the reason why chemicals react with each other to begin with is because these reactions allow atoms to obtain full outer shells.
Now you may notice that some elements in the periodic table already have full outer shells. These are called noble gases and they are placed in the most right hand side of the table (i.e. Helium, Neon, etc.). As you would expect, these noble gases are inert (do not react) because they simply do not need to. They already have a full outer shell and they are perfectly happy with how they are.
Chemical bonding
There are several types of chemical bonds that we will be looking at in this section. We will be looking at ionic bonds, covalent bonds, and metallic bonds. Before we get started though, lets get some definitions straight.
- Elements are substances made of just one type of atom
- Compounds on the other hand, are substances that are made from chemical bonds between two or more different elements.
- A mixture is a combination of two or more different substances in the absence of chemical bonds.
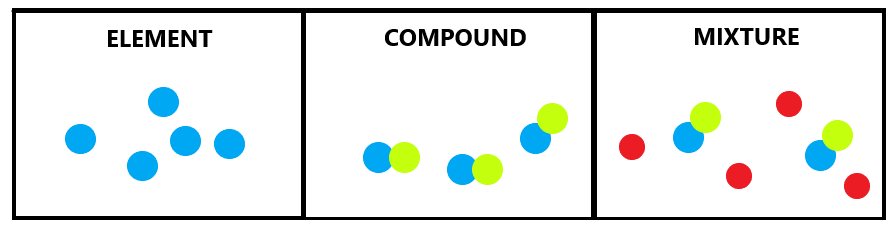
Ionic bonding [Metals & non-metals]
Background
When atoms lose or gain electrons to obtain a full outer shell, the neutral charge of the atom will be disrupted since proton number will now be unequal to the electron number. If this happens, the atom is now called an ion. The ion can have a positive charge (cation) or a negative charge (anion).
Metal atoms lose electrons to form cations and non-metal atoms gain electrons to form anions. Since cations and anions have opposite charge, they are attracted to each other via strong electrostatic forces. This is called ionic bonding: The bonding between anions and cations via strong electrostatic forces of attraction.
Here’s how it works
In ionic bonding, metallic elements will donate their outer electrons to non-metal elements that need it. Both elements will therefore achieve full outer shells and turn into cations & anions that get bonded by electrostatic forces.
Example #1
*Group 1 is simply the first column of the periodic table. Likewise, group 7 is the 7th column. More will be learnt about this in a separate topic.
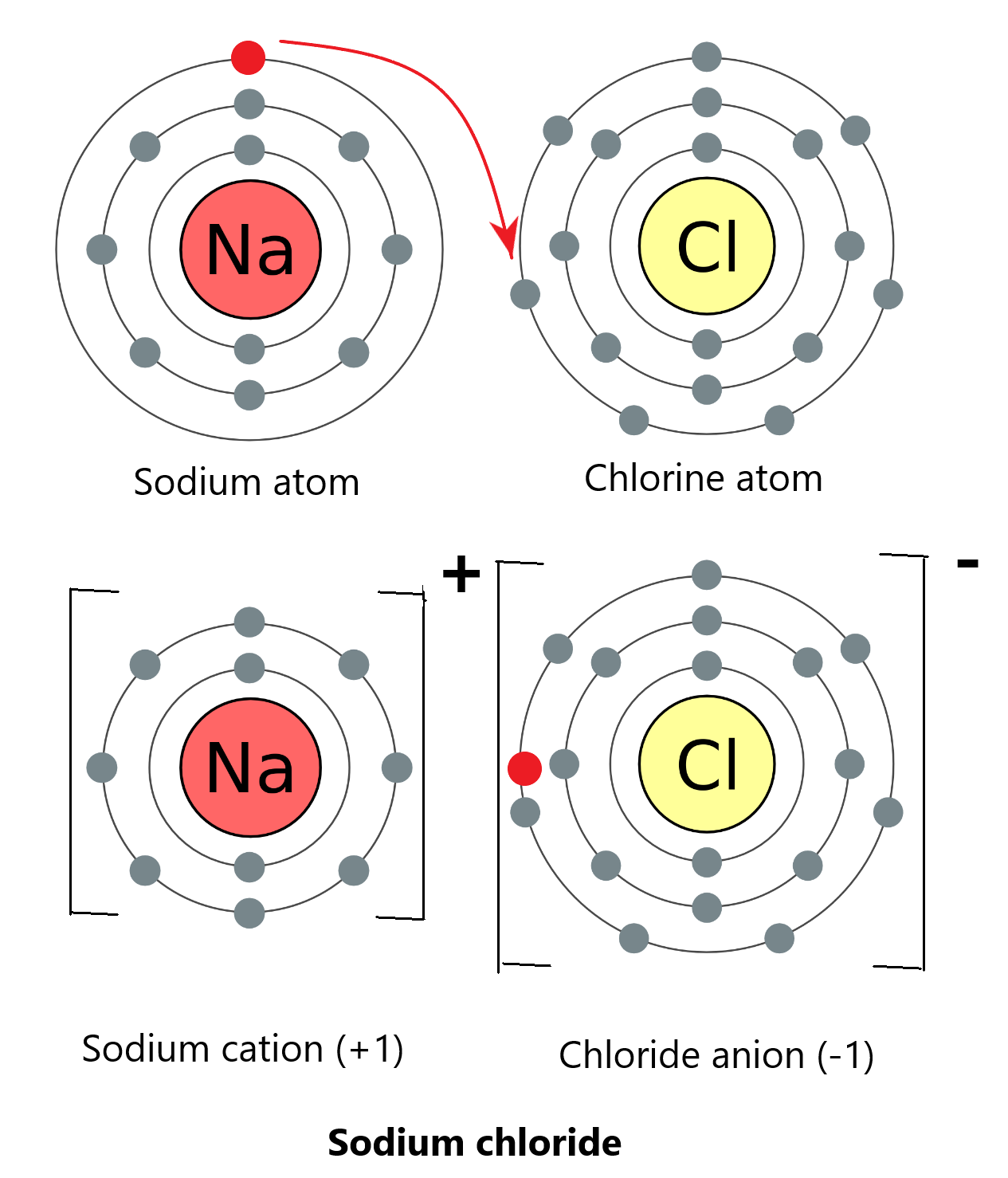
Elements of group 1 (metals) and group 7 (non-metals) in the periodic table form ionic bonds. This is because group 1 elements need to lose 1 electron to be happy, whilst group 7 elements need to gain 1 electron. This is a win-win situation! The group 1 metal will simply donate an electron to the group 7 non-metal and this will result in the formation of cations and anions that are bonded via ionic bonding.
This is an example of sodium chloride:
Example #2
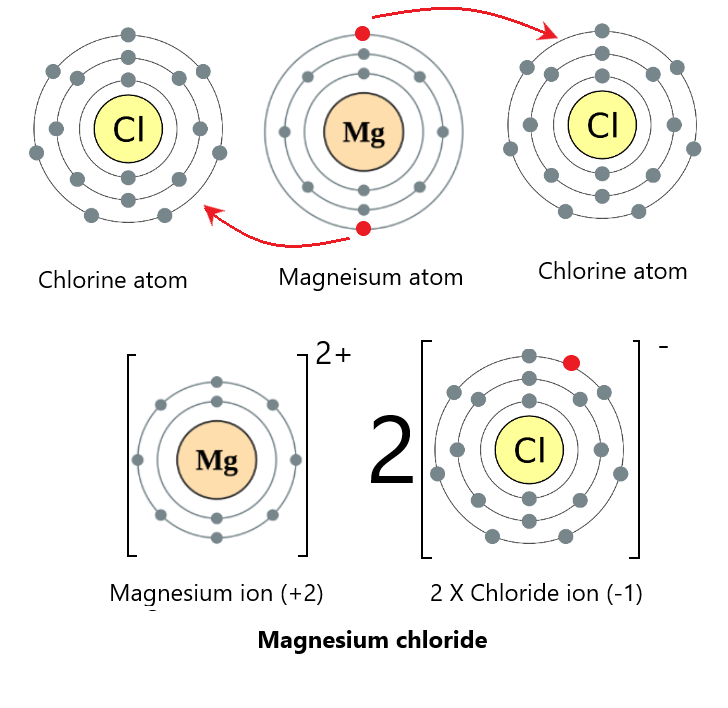
Magnesium is a group 2 element and needs to remove 2 electrons to achieve a full outer shell. Similar to the situation above, it can also form ionic bonds with fluorine (a group 7 element) by donating its electrons. The only difference is that magnesium will be donating to two chlorine atoms (giving 1 electron each).
Final structure of an ionic compound
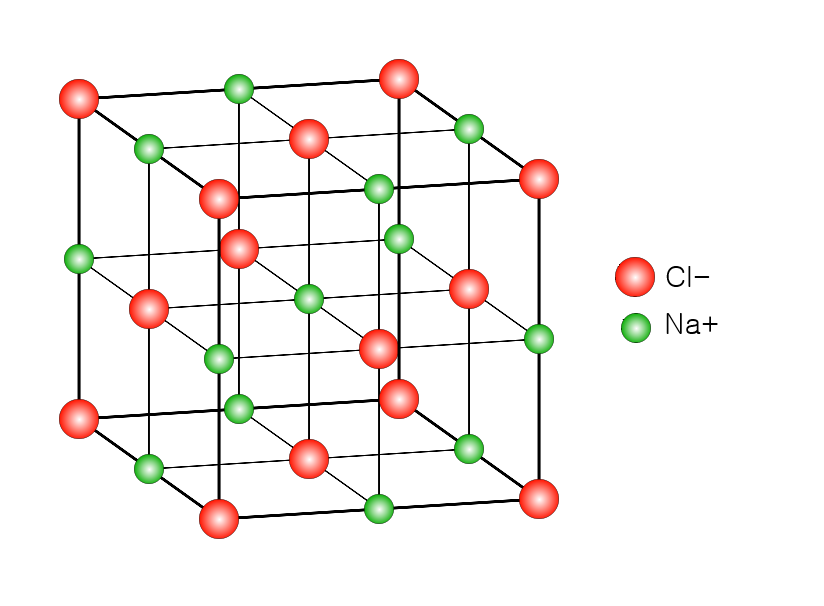
Whilst the above diagrams are used to demonstrate ionic bonding diagrammatically, it does NOT represent the final structure of an ionic compound. In fact, all ionic compounds have a 3D lattice structure.
In sodium chloride for example, many sodium cations and chloride anions will join each other in regular arrangements (called a lattice) forming a 3-dimensional structure full of cations and anions joined by ionic bonds. This is what the final structure would look like:
Covalent bonds [Non-metals & non-metals]
Atoms can achieve a full outer electron shell via sharing electrons. A pair of electrons (one from each atom) can be shared. This is a single covalent bond and it holds the two atoms together.
Please note that atoms can be bonded via a single bond (sharing a single pair of electrons), double bond (sharing two pairs) or a triple bond (sharing three pairs). Moreover, covalent bonds will ONLY exist between two non-metals.
The examples below show that by sharing electrons, all atoms in the bond successfully achieve a noble gas configuration.
Simple examples
*Only the outer electrons are shown in these examples. This is perfectly acceptable in your exams too.
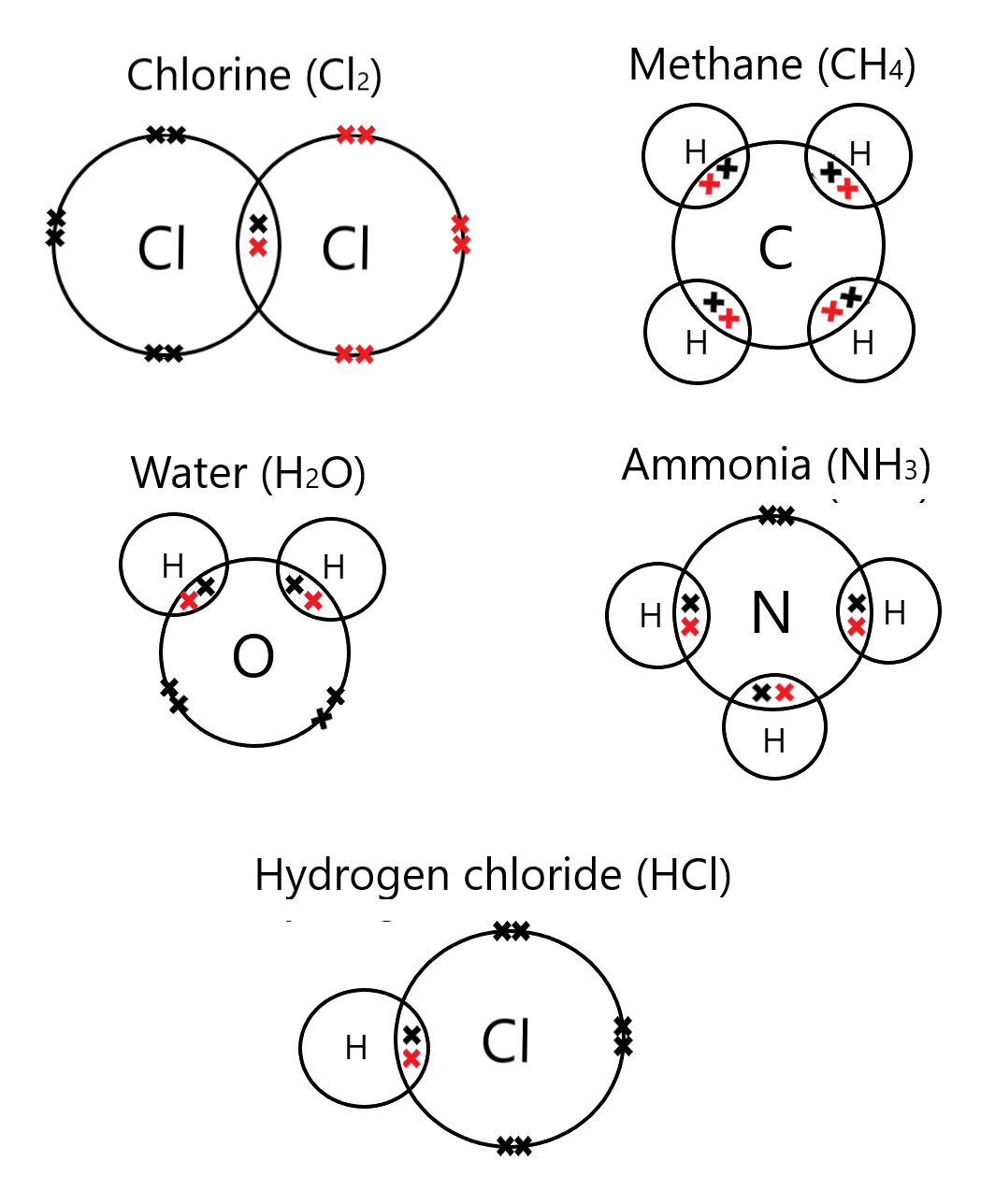
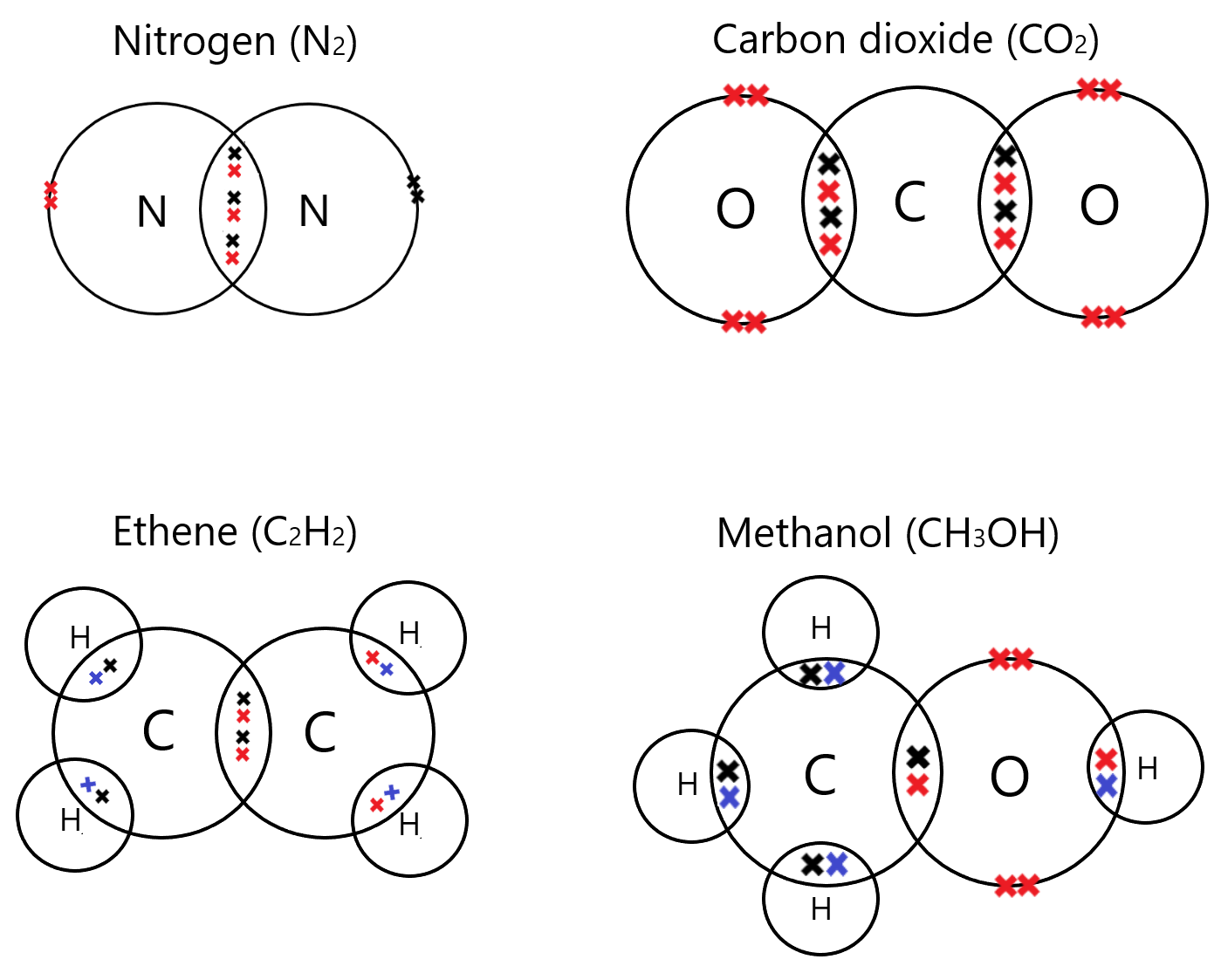
Complex examples
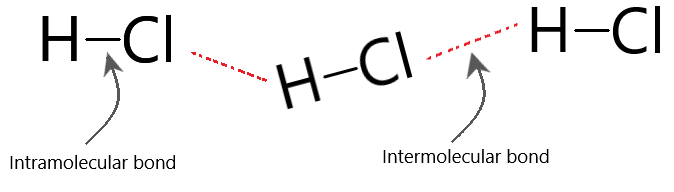
Intermolecular vs intramolecular forces
Knowing the difference between inter-molecular forces and intra-molecular forces is extremely important.
- When you are melting or boiling a substance, it is the inter-molecular forces that you are breaking, NOT the intra-molecular attraction.
Inter-molecular forces are attractive forces that exist between one molecule to another. These are usually quite weak.
Intra-molecular forces are attractive forces that exists between atoms within the molecule. These are usually extremely strong.
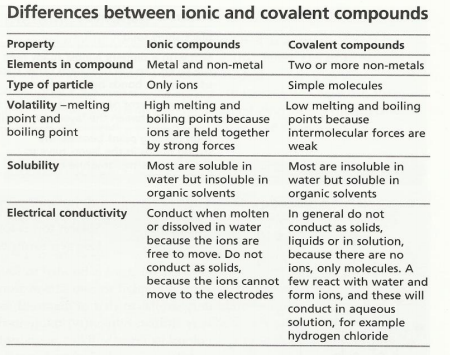
Differences between ionic and covalent compounds
This table below is from the IGCSE Hodder Revision Guide. It details the main differences between ionic and covalent compounds that CIE wants you to be aware of.
Macromolecules
Background
All of the examples of covalent molecules that we have looked at above are simple molecules. This means that atoms are bonded to one or few other atoms to make a molecule or a compound that are attracted to one another via inter-molecular forces (as described above).
Macromolecules on the other hand are giant structures made of millions of atoms all joined by covalent bonding. In other words, a huge number of atoms are joined via intra-molecular forces which are extremely powerful (also mentioned above).
There are three examples of macromolecules that you need to be aware of. We will go through each of these.
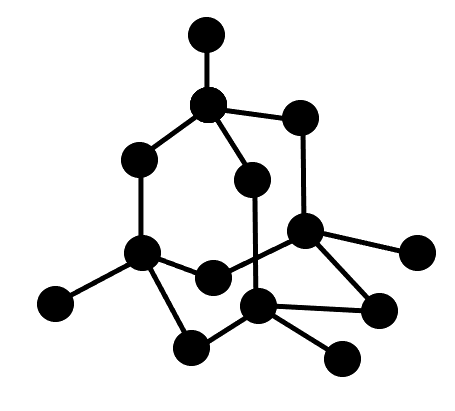
Diamond
This is a crystalline form of the element carbon. It has a three-dimensional structure in which every carbon atom is covalently bonded to 4 other carbon atoms.
This is a small part of the structure of a diamond:

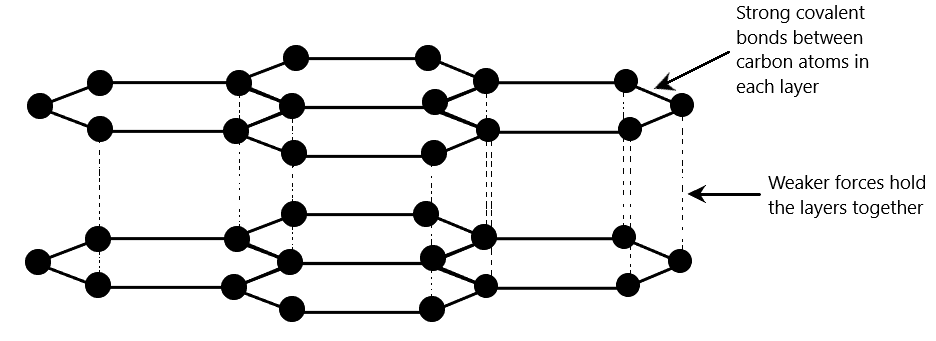
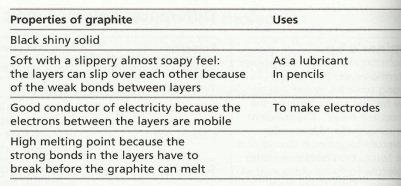
Graphite
This is another form of the element carbon. The atoms are covalently bonded in layers, with each atom is strongly bonded to 3 other atoms in the same layer.
An important thing to note is that when carbon forms 3 covalent bonds with other carbon atoms, each carbon atom will actually have a spare electron left over (you do not need to know the specifics of this).
These free electrons are called the ‘sea of electrons’ and they are free to move within the layers of graphite. It is also because of these electrons that the layers are held together (weakly).
Silicon (IV) Oxide
One silicon atom is bonded to four oxygen atoms and each oxygen atom is bonded to two silicon atoms. This structure is very similar to the structure of a diamond and consequently, the properties are also very similar. Silicon (IV) Oxide in the form of quartz exists as colourless crystals. They are very hard, have a high melting point, and they do not conduct electricity.
RED = Oxygen [2 bonds per atom]
BLACK = Silicon [4 bonds per atom]
![]()
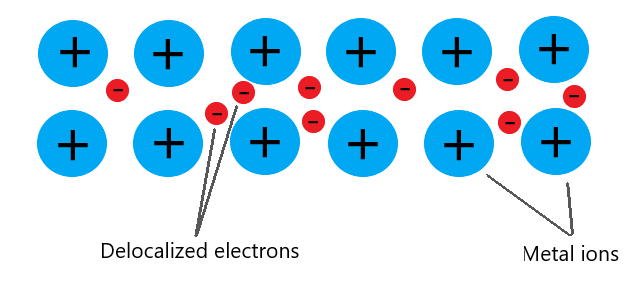
Metallic bonding
In metals, the atoms shed their outer electrons to become cations. The cations are arranged in a regular lattice structure whereas the removed electrons are delocalized and free to move throughout the structure (this is called the sea of electrons).
The lattice arrangement of cations are therefore surrounded by free electrons and since cations and electrons have opposite charge, they attract each other which bonds the structure together.
Metallic bonding is therefore defined as the electrostatic forces of attraction between cations and their surrounding sea of electrons.