Definitions
The pH scale
The pH scale is a measure of acidity or alkalinity of water soluble substances.
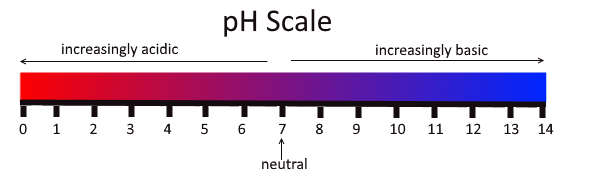
As you can see above, the scale range from 0 to 14. Water has a pH of 7 and it is therefore neutral. The lower the pH, the more acidic it is and the higher the pH the more basic it is.
- The lower the pH, the more acidic the solution is with higher the concentration of hydrogen ions (H+) in aqueous
- The higher the pH, the more basic the solution is with a higher concentration of hydroxide ions (OH-) in aqueous
pH indicators
Litmus paper, & methyl orange are used as pH indictors which allow us to determine whether a solution is acidic, basic, or neutral.
- Litmus paper
- Turns red in acid
- Turns blue in alkali (soluble base)
- No change in water
- Methyl orange
- Turns red in acid
- Turns yellow in alkali (soluble base)
- Orange in water (original colour)
Simple definition of acid & base
So what exactly are acids and bases?
The most basic definition of an acid is a substance that generates hydrogen H+ ions (or protons) in aqueous.
The most basic definition of a base is a substance that generates hydroxide ions (OH-) in aqueous.
- Bases are generally metal oxides or hydroxides
- Bases that are soluble in water are called alkalis
A more complex definition of acids and bases is associated with proton (H+) transfer. An acid is defined as a proton donor whereas a base is defined as a proton acceptor. It is required of you to be able to provide this definition in an examination.
Neutralization
Neutralization is the process by which an acid and base react to form water. Using the definitions above, it is easy to imagine this process. The H+ ions from the acid gets “neutralized” by the OH- ions from the base.
![]()
Properties of acids
Now that you understand that acids are chemicals that release H+ ions in water, lets take a look at some reactions that CIE wants you to learn.
Acid + Metal
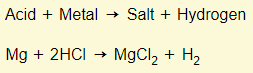
In chemistry, a salt is just another word for an ionic compound. An acid-metal reaction will always form hydrogen and the respective salt.
Acids + bases
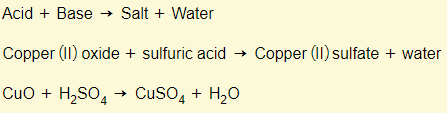
Acids + carbonates
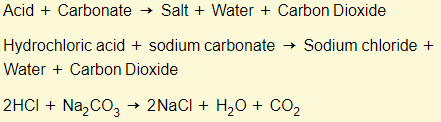
Properties of bases
There are two main reactions that you need to learn here.
Bases + acids (again)
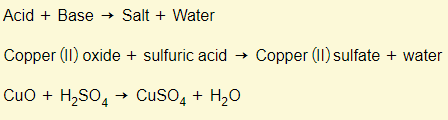
Bases + ammonium salts
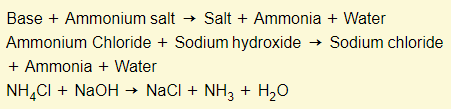
Strength of an acid or base
Strong acids and bases
Remember, acids release H+ ions in aqueous solution and bases release OH- ions. The concentration of these respective ions dictate the strength of the acid or base.
- A strong acid will have a high concentration of H+ ions (i.e. low pH)
- A strong base will have a high concentration of OH- ions (i.e. high pH)
A strong acid or base will therefore release a lot of hydrogen ions or hydroxide ions respectively. This is because the molecules are completely ionized in aqueous solution (denoted by single arrow).
Hydrochloric acid is an example of a strong acid:
![]()
Sodium hydroxide is an example of a strong base:
![]()
Weak acids and bases
- A weak acid will have a low concentration of H+ ions
- A weak base will have a low concentration of OH-
A weak acid or base will therefore release small amounts of hydrogen ions or hydroxide ions respectively. This is because the molecules are partially ionized in aqueous solution (denoted by double arrow).
Ethanoic acid is an example of a weak acid:
![]()
Ammonia is an example of a weak base:
![]()
Types of oxides
From a given oxide, CIE want you to derive whether the oxide is an acid, base, neutral, or amphoteric.
Most metal oxides are basic. Soluble bases are called alkalis and they turn litmus paper blue. Insoluble bases will not affect litmus paper. As discussed above, these basic oxides will undergo a neutralization reaction with acids.
Non-metal oxides are usually neutral or acidic
- Neutral oxides such as water, nitrogen (II) oxide, and carbon monoxide do not react with acids or bases
- Acidic oxides such as sulphur dioxide, sulphur trioxide, carbon dioxide, and oxides of phosphorus will turn litmus paper red and neutralize bases
Amphoteric oxides react with either a base or acid to form salt and water. This means that these oxides have the properties of a base and acid. Examples are zinc oxide/hydroxide and aluminium oxide/hydroxide. You need to learn these two examples:
Behaving as a base
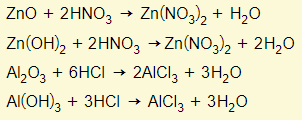
Behaving as an acid
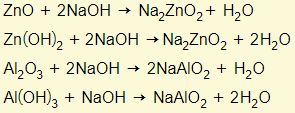
Salt preparation
- Titration
- This is used to prepare a soluble salt from a soluble base (i.e. alkali) and an acid. This method is used to make salts of group 1 metals and ammonium salts.
- Neutralization of insoluble base by acid
- An excess of the base is added to an acid, and the excess is removed via filtration. The filtrate is partially evaporated to obtain crystals of the salt. Soluble salts of most metals that are not group 1 are made by this method.
- Metal reacting with acid
- This method is basically the same as above. It can make magnesium, zinc, aluminium, and iron (II) salts. However it cannot be used to prepare salts of reactive metals (such as sodium and potassium) due to the violent reaction.
- Preparing insoluble salts via precipitation
- Here is a list of the insoluble compounds that you need to know. Salts not on this list are considered soluble.
- All carbonates except aluminium carbonate and all group 1 carbonates
- All hydroxides except calcium, strontium, barium, and group 1 hydroxides
- Barium, calcium, and lead sulphates
- All chlorides, bromides, and iodides of silver and lead
- Here is a list of the insoluble compounds that you need to know. Salts not on this list are considered soluble.
To make barium sulphate for example (an insoluble salt), two solutions must be mixed. One solution must contain a soluble barium salt (i.e. barium chloride) and the other containing a soluble sulphate (i.e. sodium sulphate). The precipitate of barium sulphate is filtered off, washed, and dried.
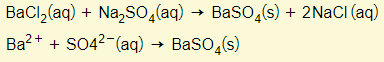
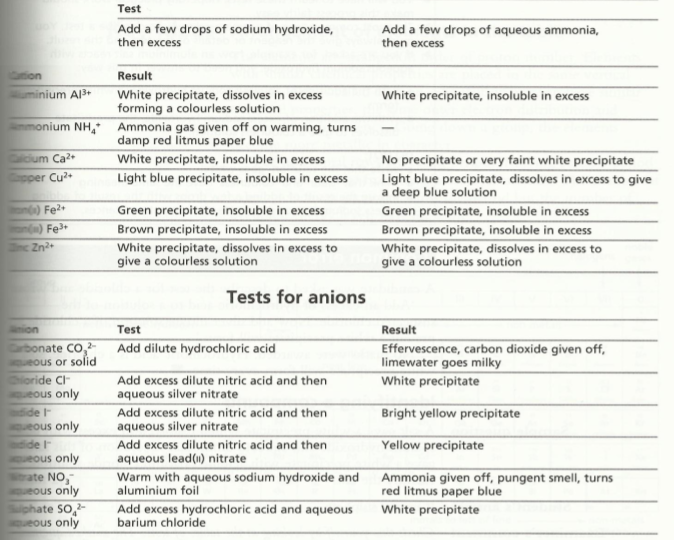
Ion & gas identification
You should be familiar with ion and gas identification as a part of your practical work. But this table will assist you: