Physical vs chemical changes
It is important for you to understand the difference between chemical and physical changes. Some changes are obvious, but there are some basic ideas you should know.
Physical changes are usually about physical states of matter . Chemical changes happen on a molecular level when you have two or more molecules that interact. Chemical changes happen when atomic bonds are broken or created during chemical reactions .
Collision theory
At an atomic level, a chemical reaction will occur when two conditions are met. Two particles need to collide and they must have enough energy to react. This means the reaction rate will depend on these two factors: Collision rate & particle energy.
If two atoms collide but they don’t have enough energy, then the reaction will not occur. If the particles have enough energy but they don’t collide, then again, the reaction will not occur.
It is very important to realize that there are certain things such as concentration, pressure, temperature, catalysts, and particle size that affect the collision rate & particle energies, and therefore directly affect the chemical reaction rate.
Factors affecting rate of reaction
We briefly mentioned above the several factors that affect reaction rates. We will be going through each of these in a bit more detail.
Firstly however, It is important to note that the best definition for the rate of reaction is:

This means that the rate is dependent on concentration (i.e. amount of substance in a specified volume) rather than just the amount of substance.
Concentration
When the concentration is increased, the rate of reaction is also increased due to higher collision rates (since there are more particles per unit volume).
Pressure
The pressure only affects reactions with gases. An increased pressure means gas molecules are closer together. This increases the collision rate and thus the reaction rate.
Temperature
When the temperature is increased, the rate of chemical reaction will increase due to larger amounts of energies of individual particles and a higher collision rate (since particles are moving quicker).
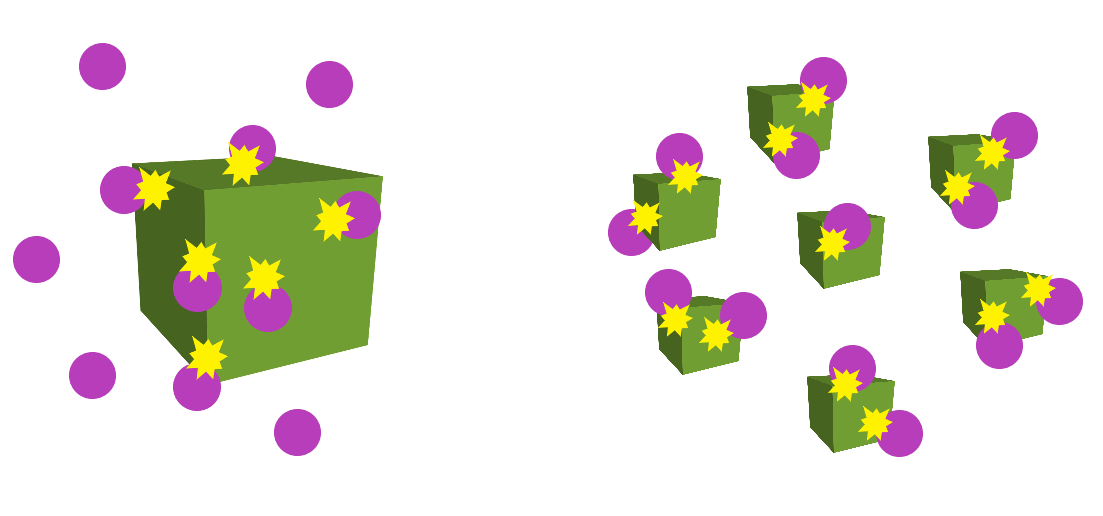
Particle size
This only affects reactions involving solids. Smaller particle sizes mean that there is a larger surface area for collisions to occur, which in turn, increases the reaction rate.
This diagram below demonstrates this concept well:
Catalysts
A catalyst increases the rate of a reaction but remains chemically unchanged. Enzymes are biological catalysts.
Experimental methods
CIE expects you to understand how to devise suitable experiments to investigate each of the above variables on reaction rates.
Most experimental techniques should be learnt in the lab as a part of your curriculum. We will touch on the details of experimental techniques in a separate section.
Photochemical reactions
Reduction of silver (I) Halide
This is the basis of photography. A photographic film i coated with a layer of silver (I) bromide. When exposed to light, silver ions accept electrons from bromide ions and form silver atoms. This is called reduction (more details down the page)
![]()
Parts of the film that have been exposed to light turn black, while unexposed portions remain white, The rate of reaction depends on intensity (i.e. brightness) of the light.
Photosynthesis
Green plants make carbohydrates via this reaction.:

The reaction is catalysed by chlorophyll (the green pigment in plants) and occurs only in sunlight. Again, the rate of reaction is dependent on light intensity.
Reversible reactions
A reversible reaction is a chemical reaction where the reactants form products that, in turn, react together to give the reactants back.
When hydrated copper (II) sulphate is heated, it decomposes. This is the forward reaction which is endothermic.
![]()
When the products are cooled and mixed, the reverse reaction occurs. This reaction is exothermic.
![]()
Therefore the overall reversible reaction can be written into one equation:
![]()
Equilibrium
As we saw above, in a reversible reaction, the reactants make the products and the products make the reactants.
Eventually, the reaction will reach an equilibrium whereby the rate of the forward reaction and the reverse reaction are equal. This means that the concentrations of reactants and products will stay exactly the same (unless the conditions are changed).
The concentrations of reactants and products in an equilibrium is called the position of equilibrium.
- If the position of equilibrium moves to the right, it means that in the “new” equilibrium, the concentration of products has increased whereas the concentration of reactants has decreased.
- If the position of equilibrium moves to the left, it means that in the new equilibrium, the concentration of reactants has increased and the concentration of products have decreased.
For example:
![]()
If the equilibrium shifts to the RIGHT that means more C is being made from A and B. Therefore, the concentration of C increases whilst the concentration of A and B decreases.
If the equilibrium shifts to the LEFT that means more A and B is being made from C. Therefore, the concentration of C decreases whilst the concentration of A and B increases.
There are certain conditions that affect the position of equilibrium.
***Very important note***
The position of equilibrium will shift in the direction that OPPOSES the change in condition.
For example, increasing the concentration of product C will shift the equilibrium to the left (to try and reduce the concentration). Bear this in mind when reading through the separate conditions below.
- Concentration
- Increasing the concentration of the product will shift the equilibrium to the left (to reduce the product concentration). Decreasing the product concentration will shift the equilibrium to the right (to produce more).
- Increasing the concentration of the reactant will shift the equilibrium to the right. Decreasing the reactant will shift it to the left.
- Temperature
- In a reversible reaction, one reaction is exothermic and the other is equally endothermic.
- Increasing the temperature will therefore shift the equilibrium towards the endothermic reaction (to reduce heat)
- Decreasing the temperature will shift the equilibrium towards the exothermic reaction (to increase heat)
- Pressure
- This factor is only relevant for reacts that involve gases. Please refer to this example
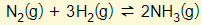
- Note that the total number of gas molecules in the LHS = 4 and the RHS = 2
- Increasing the pressure will move the equilibrium to the side with a smaller number of gas molecules (i.e. Right in this example)
- Decreasing the pressure will move the equilibrium to the side with a larger number of gas molecules (i.e. Left in this example).
- This factor is only relevant for reacts that involve gases. Please refer to this example
- Catalysts
- CIE loves to trick you with this so be careful
- Catalysts do NOT affect the position of equilibrium. It affects the rate of reaction only.
Redox
Oxygen gain/loss
Redox is shortened for ‘reduction’ and ‘oxidation’. This can be explained via the gain or loss of oxygen.
- Oxidation is the gain of oxygen
- Reduction is the loss of oxygen
For example:
![]()
In the equation above, CuO has been reduced because it has “lost” an oxygen to become Cu.
Meanwhile, the hydrogen has been oxidized because it has “gained” an electron to become H2O.
Electron transfer
The concept of reduction and oxygen can also be explained in terms of electron gain or loss.
- Oxidation is the loss of electrons
- Reduction is the gain of electrons
For example:
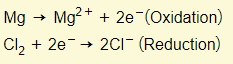
In the example above, the magnesium atom loses electrons to become an ion so therefore it has been oxidized.
The chlorine molecule on the other hand, gains two electrons to become chloride ions and therefore it has been reduced.
I’ve explained some important concepts here in this video