Measurement
CIE wants you to demonstrate the ability to name the appropriate apparatus for the following: Time, temperature, mass, volume:
- Time
- Digital stop watch
- Measures up to 0.01s
- Digital stop watch
- Temperature
- Mercury-in-glass thermometer
- Measures up to nearest °C
- Mercury-in-glass thermometer
- Mass
- Electric top-pan balance
- Measures up to 0.01g
- Electric top-pan balance
- Volume
- Beaker
- Used to estimate liquid volume
- Measuring cylinder
- Measures up to 0.1cm3 (More accurate than beaker)
- Pipette
- Measures fixed volumes of liquids accurately (i.e. 20cm3)
- Measures up to 0.1cm3
- Burette
- Used for measuring variable volumes of liquids accurately
- Measures up to 0.1cm3
- Beaker
Criteria of purity
The purity of a substance is defined as the degree to which a substance is undiluted or unmixed with other material. A pure substance therefore would be made of a single substance.
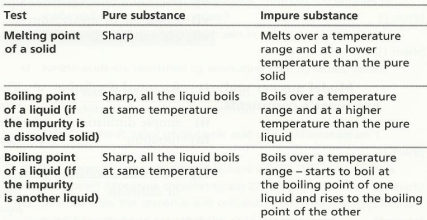
Purity assessment from melting point/boiling points
The melting point of a substance is the temperature in which the substance melts. Similarly, the boiling point of a substance is the temperature at which it boils.
Interestingly, the boiling point and melting point of a substance can give us an indication of how pure it is. The table below summarizes this quite well (Hodder IGCSE Chemistry Revision Guide):
Paper chromatography
Paper chromatography is a separation technique that is used to separate and identify the components in a mixture.
How it works is fairly easy. Lets imagine you have an unknown liquid (Liquid A). You want to find out whether or not this liquid is impure (i.e. a mixture) and if so, how many substances are in this mixture and what exactly are they?
Firstly, you simply get a drop of liquid A and place it onto the chromatography paper. You then draw a horizontal line marking that drop (you’ll see why this is important later).
You then set up the chromatography paper inside a beaker so that the bottom of the paper is just immersed inside the solvent (propanone or water). An example of this set up may look like this:
As time passes, the solvent will travel up the chromatography paper. As the solvent moves up, the sample spot of liquid A will dissolve in the solvent. If liquid A was a mixture, the various substances inside the mixture will begin to separate because they have different solubilities. Some substances will travel up the paper slower than others and reach a different end point. The end result may look like this:
In this particular example, it is clear that the ink spot (liquid A) is a mixture. Why? Because you can see that it has separated out into 3 different components (green, purple, and yellow. If liquid A was pure then you would only see one component.
*If liquid A was colourless, then the process can be carried out exactly as before but a locating agent will be required to “locate” all the separated spots later in order to measure the Rf values
Finally, since we know that liquid A is a mixture, we can actually determine what each of the substances are exactly. To do so, we need to calculate the Rf value of each of the separated components on the chromatogram.
Rf Value = Distance traveled by spot / Distance traveled by solvent
So lets go back to the example above:
The Rf value for:
- Green spot: 5 / 6 = 0.83
- Purple spot = 3/6 = 0.50
- Yellow spot = 1/6 = 0.17
All substances have a unique Rf value, and therefore you will be able to find out what exactly the substance is if you have a reference table. In an examination, they will always provide you with this.
Methods of purification
Filtration, centrifuging, decanting
All of these these methods are used to separate an insoluble solid from a liquid.
- Filtration is carried out by pouring the mixture into a funnel covered by a filter paper. Whilst the liquid will pass through the filter, solids will get caught, thereby separating them.
- Centrifuging is a technique which uses a spinning tube. The spinning generates a strong centripetal force which causes denser materials (i.e. solids) to travel towards the bottom of the centrifuge tube at a faster rate than normal gravity.
- Decanting is the simple process of letting insoluble solids settle in the liquid before gently pouring it out later.
Evaporation
This is a simple process of separating the crystals of a solute from a solution. Simply let the solvent evaporate off and it will leave the solids behind.
Crystallization
This technique is used to separate two soluble solids from a solution (given that they have different solubilities). It works by dissolving the two solids in minimal water, and then slowly cooling it. The less soluble salt will crystallize first.
Simple distillation
This method is used to separate a volatile liquid (easy to evaporate) from a solution with a non-volatile solid. For example, salt water can be purified using this method. The equipment set-up is as follows:
The heat causes the water to vaporize, leaving the salt behind. The water vapor then turns back into (pure) water as it passes through the condenser (which cools the vapor down).
Fractional distillation
This method is used to separate the different liquids from a liquid mixture. For example, a mixture of water and ethanol can be separated using this method.
Fractional distillation works by using the fact that different liquids have different boiling points. It is a bit more complicated than simple distillation but here is how it works:
For arguments sake, lets say the round flask contains a mixture of liquid A (boiling point 50°C) and liquid B (boiling point 100°C).
As you heat the flask with the Bunsen burner, the temperature of the flask and the fractionating column will begin to rise. It is really important to understand here that the bottom of the long column is always going to be hotter than the top. This is simply because we are heating from the bottom and it takes time for the heat to rise up.
So lets imagine the round flask hits 50°C. What do you think will happen? Well, liquid A will start to boil in the flask but the vapour won’t get far in the column before cooling back down into liquid (back into the flask). This is because of the temperature gradient in the column.
The very bottom of the column might be 50°C but the top will be cooler than that, meaning the gas will just condense back to a liquid before reaching the top. Therefore the main point to take away here is that the gas can only go up as far as the temperature in the column allows it to.
Eventually however, the column will heat up sufficiently. When the top of the column reaches 50°C, the vapour of liquid A will reach all the way to the top and get condensed into liquid by the condenser. Once all of liquid A has entered the flask, we simply need to replace it with an empty one.
The column will then continue to heat up. Eventually, the top of the column will hit 100°C whereby liquid B will reach the top of the column and become liquefied by the condenser and into our empty flask.
By utilizing the differing boiling points of liquid A and B, we have successfully separated it from the original mixture.
Purification summary
This is a handy summary from Hodder IGCSE Chemistry revision guide: